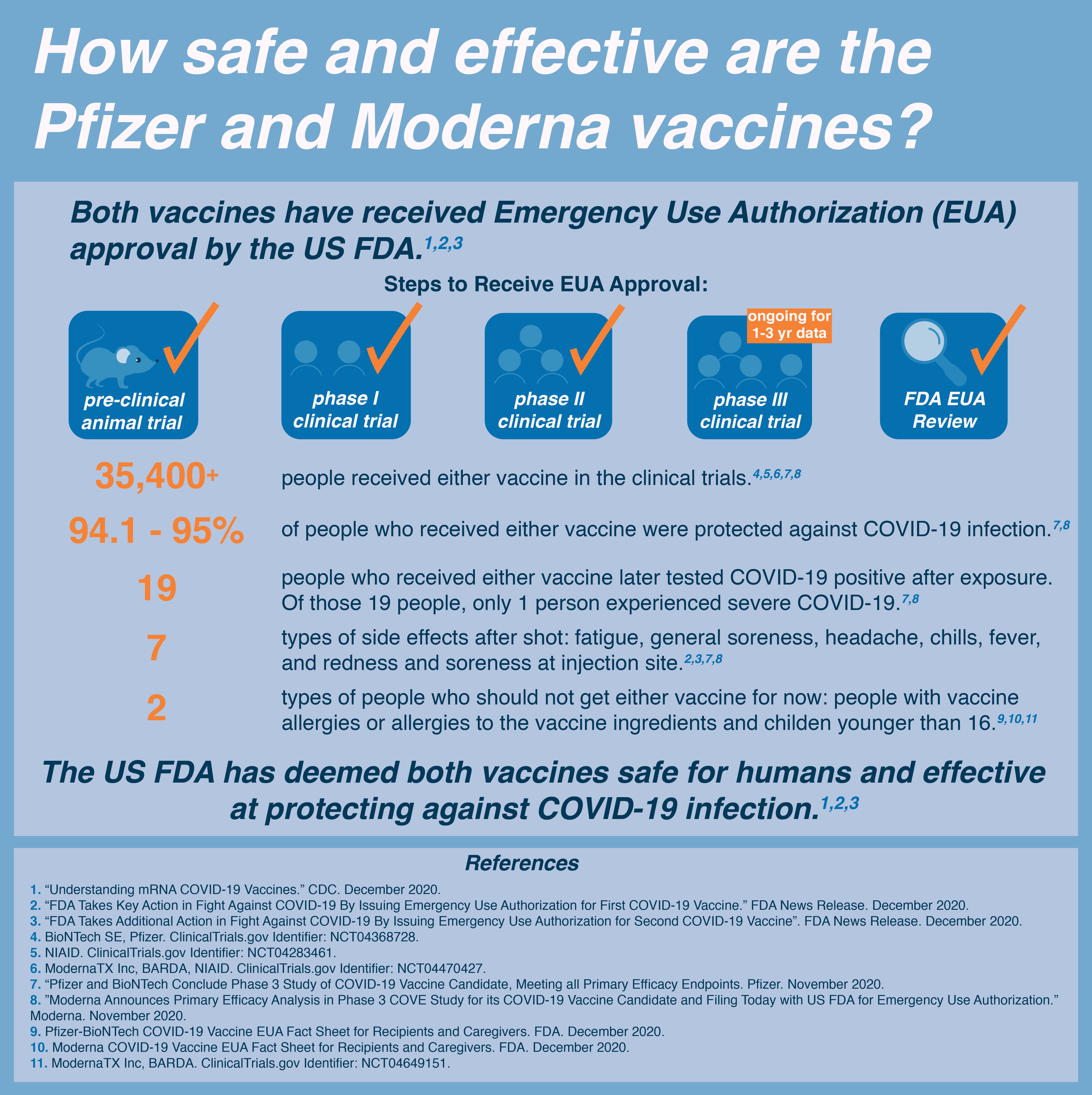
Safety and efficacy of the COVID-19 vaccines
Big thanks to Stanford Biosciences PhD & MD-PhD students–especially Michelle Drews, Molly Lucas, Daniel Wesche, Eva de la Serna, Evan Boyle, Aofei Liu, Megan Garland, Shenandoah Wrobel, Erin Sanders, and Sergey Stavisky–for contributing text, citations, images and thoughts that I’ve compiled into this post.
I’m delighted to share that my mother received the Moderna COVID-19 vaccine yesterday, and that my sister will receive the Pfizer vaccine on Monday. As soon as I’m eligible to receive one, I’m excited to follow in their footsteps. I miss seeing my friends, I find masks uncomfortable, I want to travel again, and I have a newfound craving to grab a drink in a packed nightclub. Wouldn’t it be great if someday soon we could go back to all these things because enough people got vaccinated?

I was recently forwarded an article from the Weston A Price Foundation that on its surface seems well-researched and authoritative, and raises a contrarian perspective on the vaccine. I know how terrifying this year has been, and how a botched response to COVID-19 has led to skepticism of our government leadership. And I can appreciate how the uncertainty in the early days of the pandemic has led to skepticism of our scientists. In hindsight, the crisis-induced recommendation from Dr. Anthony Fauci for American citizens to refrain from wearing facemasks in an effort to preserve dwindling Personal Protective Equipment (PPE) for first responders was unfortunate in context of later evidence on the efficacy of masks in reducing transmission of the virus.
We talk about “uncertainty” in science just as additional information; understanding the bounds of your confidence / uncertainty is critical to evaluating a model of what’s going on. I think the lay public sometimes interprets scientific uncertainty as if it meant “we have no clue”, and therefore stop trusting anything coming from that source. Unusually this year, the public got to witness the full machinery of the scientific process, in real-time, with unprecedented transparency as scientists publicly offered peer review of new findings on Twitter or to the media. As a result, the public saw highly publicized disagreeements between scientists as interpretations are challenged and information that represented a best-approximation at the time is replaced with newer information that refines our understanding. Sometimes scientists get things wrong, and others notice & correct. Eventually, with enough time and attention, the scientific process converges closer and closer to an understanding that satisfies all the data.
The email I’m about to share contains misleading framing and dangerous misinformation on the safety and efficacy of the Pfizer-BioNTech COVID-19 Vaccine. I circulated this email to Stanford’s internal Biosciences email Listserv, and compiled the below line-by-line rebuttal of the email from my peer’s responses. Before we jump in, since this blog post is rather lengthy, let’s begin with a recommendation distilled from the CDC’s Interim Clinical Considerations:
If you are 16 years of age or older, and do not have history of severe allergic reaction to vaccines or other injectables, you should get vaccinated as soon as it is made available to you.
Most of the concern I’ve heard about the vaccine stems from worries about the symptoms. The reason people get symptoms from many vaccines is actually a sign that it’s working–your body is reacting to the vaccine, and in doing so will be prepared to fight off the virus if you run into it later. I think many people view side effects as meaning the vaccine is not working, but that’s not necessarily the case. Ultimately, medical treatments aren’t something you would take recreationally, as they’re rarely without risk. A treatment is the lesser of two evils. There’s a saying–don’t let perfection be the enemy of productivity–we get a lot done with modern medicine even though it’s always imperfect.
Without further ado, here’s the email and rebuttals.
From: Weston A Price Foundation info@westonaprice.org
Subject: Important points about new vaccines
Date: December 17, 2020 at 10:35:35 PSTCOVID-19 VACCINES: IMPORTANT POINTS
MINOR IMPACT: Vaccine manufacturers claim that Covid-19 vaccines are 95 percent “effective,” but the FDA is allowing companies to define effectiveness as “prevention of mild symptoms.” The studies are not designed to detect a reduction in outcomes such as severe illness, hospitalization or death.1,2
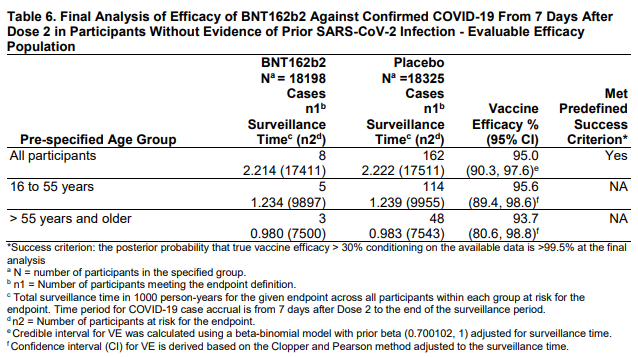
The trials are designed as such: give a bunch of people the vaccine or a placebo (they don’t know what they got) and follow them to see if they get COVID-19. Efficacy is based on how many in the vaccine group get COVID-19 vs how many in the placebo group get COVID-19. For the Pfizer vaccine there were 169 people in the placebo group that got COVID-19. There were 9 in the vaccine group. The trials are set up this way because reduction in cases is a reasonable outcome, and to use reduction in severe illness, hospitalization, and death as outcomes would take far longer to get a statistically useful number of cases. That doesn’t mean severeness of illness isn’t taken into account. In the placebo group, 9 patients developed severe COVID-19. In the Vaccine group, only one did. See the NEJM article for all details.
For individuals who develop severe symptoms, the vaccine is not a remedy.
Nobody in the medical community is arguing it is a remedy. Vaccines are preventative measures designed to teach the immune system to fight a pathogen before it sees the pathogen. If the immune system doesn’t “learn” (and this happens in some patients) the vaccine itself is not a therapy.
Instead, nutritional and oxidative support can help keep the illness from going into ’overdrive.’3
Proper nutrition and support are essential in giving patients the best chance to fight any illness. However, antivirals and steroids have also shown to be effective therapies that are currently standard of care.
EXPECT ADVERSE REACTIONS: Participants in every Covid-19 vaccine trial have reported adverse reactions including high fever, chills, muscle pains and headaches.4-6
Side effects can occur with all vaccines however it’s important to contextualize them. Pain / redness around the injection site is reported as a side effect but it is an expected one - you are sticking a needle into a patient after all and that hurts! Fever, chills, and a general flu like symptoms are also expected side effects. You take a vaccine to trigger an immune response - and a fever / chills etc are part of a normal immune response. They are signs that the vaccine is doing its job and your immune system is responding appropriately.
Finally with any side effect profile I [MD-PhD friend writing here] personally make a point to warn patients about the risks in two ways. The first thing I tell them about is the common side effects - the ones that they are likely to experience. For this vaccine that is fatigue and headaches (~55% of patients experienced; also 24% of placebo patients though which might be more of a comment on the stressful times we’re living in), and fever (~15% of patients).
The next thing I tell them about are the serious side effects - the RARE but important things I can’t leave out just in case the worse happens. When discussing the scary, rare side effects it’s important to be armed with exactly how many patients actually get those rare side effects. For this vaccine 64 participants (0.3%) reported lymphatic swelling. There were 4 severe adverse effects - 1 shoulder injury due to administration, 1 severe lymphadenopathy, 1 ventricular arrhythmia, and 1 right leg paresthesia. There also seems to be a chance of a serious allergic response in people with allergies.
Now what people don’t really appreciate about clinical trials is that they’re very strict with side-effect reporting. Anything a patient reports goes down as a potential side effect, even if the cause of their nausea is actually a bad plate of sushi or something entirely unrelated. In the trial, two patients died - both from cardiovascular issues. But four placebo patients also died, two from unknown causes, one from a stroke, and one from a heart attack. None of the investigators or outside observers think the vaccine was the cause. But safety monitoring continues.
Some have even reported severe reactions that required hospitalization and invasive treatment.
This is true, but missing important context. Four people out of 21,720 for the Pfizer vaccine reported severe reactions.
According to the FDA, potential long-term effects may include Guillain-Barré syndrome, brain swelling, muscle weakness and paralysis, convulsions and seizures, stroke, narcolepsy, shock, heart attack, autoimmune disease, arthritis and joint pain, multisystem inflammatory syndrome in children, and death.7
First, regarding Guillain-Barré syndrome, this is a risk we include for all vaccines - the pathogenesis of GB is still poorly understood. All of the remaining potential long-term effects are also risks for patients with COVID-19.
Some UK health workers have experienced anaphylactic shock after receiving one dose of the approved vaccine.8
This is true - and currently medical providers are being very cautious about giving the vaccine to people with a known history of severe allergic reactions to vaccines or injectables.
WON’T PREVENT COVID-19: An FDA Pfizer briefing paper published December 10, 2020 revealed 43 percent more suspected cases of Covid-19 in the vaccinated group than in the placebo group within seven days of vaccination.9
Hard to tell if this is deliberate misinformation or just paranoia. The full quote says: “Among 3410 total cases of suspected but unconfirmed COVID-19 in the overall study population, 1594 occurred in the vaccine group vs. 1816 in the placebo group. Suspected COVID-19 cases that occurred within 7 days after any vaccination were 409 in the vaccine group vs. 287 in the placebo group. It is possible that the imbalance in suspected COVID-19 cases occurring in the 7 days post-vaccination represents vaccine reactogenicity with symptoms that overlap with those of COVID-19.”
It is notable that 7 days after the first dose patients and controls are roughly even - it’s really only after the about 14 days that you start seeing protection. The second dose is given at day 21. This result makes a lot of sense if you understand immune reactions. It takes about 7 days for you to build up a robust antibody response to a foreign pathogen because it takes time for the pathogen to encounter the right immune cell and for that cell to get activated and expand and mature.
NO LIABILITY: Covid-19 vaccine manufacturers will be protected from all liability—if you are injured, you cannot sue.10 Manufacturers will have complete indemnity even though all previous attempts at creating coronavirus vaccines caused harm and never advanced to regulatory approval.11
In the United States, vaccine injury is compensated by the US government. Because vaccines are widely administered, even fairly rare (<1%) moderate side effects could easily bankrupt a manufacturer if it could be sued. To promote vaccine development, the government assumes risk from vaccines. This means that any injuries (e.g. anaphylaxis, extreme fevers) are reviewed by the VICP and treatments covered accordingly.
If anything this is preferable to manufacturer liability because the evidentiary threshold is lower, and you don’t have to deal with as many legal hurdles and appeals.
WILL NOT END RESTRICTIVE MEASURES: Dr. Anthony Fauci of the National Institutes of Health acknowledges that the vaccines may prevent symptoms but will not block spread of the virus, so vaccine recipients will still need to wear masks, practice social distancing and avoid crowds.12,13
When you read the two articles referenced here, neither of them says that the vaccine will not block the spread of the virus. What Fauci said is that it won’t be a switch. Sure, to end restrictive measures we need to achieve something like herd immunity. Which means upward of 50% of the population vaccinated, perhaps upward of 70%. For a 2-dose vaccine that means 300M to 450M doses of vaccine distributed just in the US alone, which is A LOT. First allocation for US is 100M from Pfizer vaccine. It’ll take until the summer/fall until enough doses can even be produced to cover everyone.
As for if the vaccines prevent spread or just symptoms, the jury’s out on that one. It may prevent, we simply don’t yet know. However, if you’re vaccinated, even if someone spreads it to you, you still have a 95% chance of not getting any symptoms. Sounds like a good deal to me!
NOT NECESSARY: According to the CDC’s current best estimate, the “infection fatality rate” (IFR) for Covid-19 is less than 1 percent for people age 69 and younger, including a .003 percent IFR for children and adolescents.14
There are many people over 69 in the US population, and, sadly, many more with diabetes, heart disease, and other conditions that make them at high risk for severe consequences. Those of us fortunate enough to be low risk can give it to our elders and immunocompromised friends.
In addition, the focus on death leads to terrible, terrible misconceptions that the illness is mild. The average duration of a COVID-19 infection is ~2 weeks. That’s more than your average flu, and still people get vaccinated for the flu. It’s estimated that 1-in-10 to 1-in-5 become long-haulers, i.e., persistent symptoms. Here’s a good summary, with many shocking statistics like “35% of 274 symptomatic respondents reported not having returned to their usual state of health 2 weeks or more after testing, including 26% among those aged 18-34 years (n = 85).”
It’s much worse among those who are hospitalized. 87% are not fully symptom-free after 60-day follow-up. Also, 5-15% of all COVID-19 cases are hospitalized. I’ll take a vaccine to avoid a 2-week hospital stay any day…
COULD MAKE YOU STERILE: Two prominent doctors, including the ex-head of Pfizer’s respiratory research, warn that Covid-19 vaccines contain a spike protein called syncytin-1, vital for the formation of the placenta.15 If the vaccine triggers an immune response to this protein, then female infertility, miscarriage or birth defects could result.
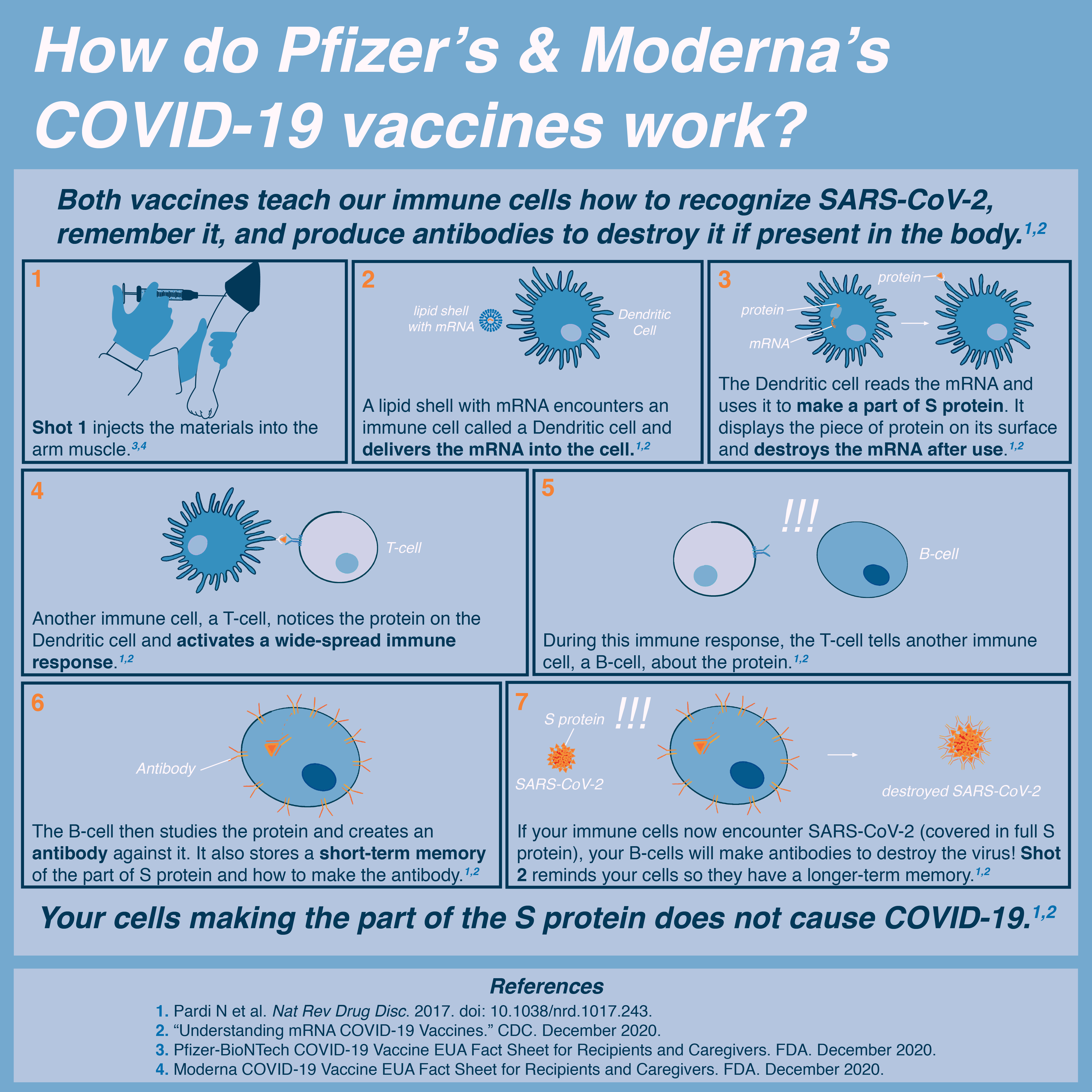
The COVID-19 vaccine does not contain syncytin-1. The vaccine contains mRNA that transiently teaches the body to make the COVID-19 spike protein, a viral protein.
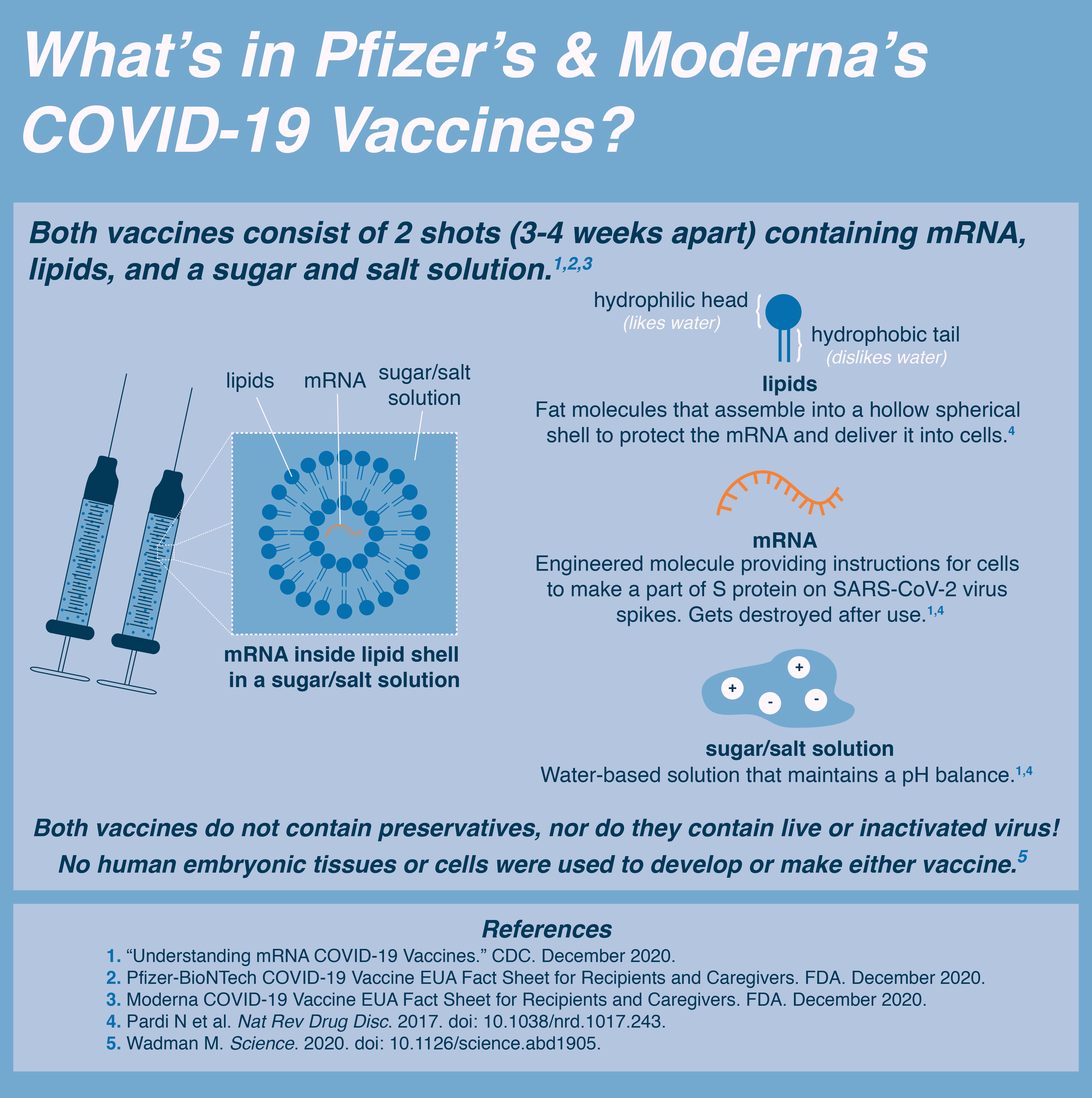
The COVID-19 spike protein shares some similarities to the human protein syncytin-1, however the similarity is too small to cause a cross-immune reaction according to Dr. Ian Jones of the University of Reading. If cross reactivity from COVID-19 and syncytin-1 were a concern we would see a much higher rate of miscarriage in pregnant women infected with COVID-19 or previously infected with COVID-19. We do not see this according to Dr. Mary Minkin - a Yale OB/GYN. Additionally, the scientists making these claims: Dr. Wolfgang Wodarg and Dr. Mike Yeadon have been previously criticized for making misleading claims about the virus and vaccine.
Finally, here’s the end of the email, with their citations:
FOR FURTHER INFORMATION (including printable flyers): https://www.westonaprice.org/covid-19-vaccines-important-points/
- Doshi P. Will covid-19 vaccines save lives? Current trials aren’t designed to tell us. BMJ. 2020;371:m4037. https://www.bmj.com/content/371/bmj.m4037.
- Haseltine WA. Covid-19 vaccine protocols reveal that trials are designed to succeed. Forbes, September 23, 2020. https://www.forbes.com/sites/williamhaseltine/2020/09/23/covid-19-vaccine-protocols-reveal-that-trials-are-designed-to-succeed/?sh=5da0663d5247.
- Brownstein D, Ng R, Rowen R et al. A novel approach to treating COVID-19 using nutritional and oxidative therapies. Science, Public Health Policy, and the Law. 2020;2:4-22. https://ozonewithoutborders.ngo/wp-content/uploads/2020/07/Novel-Approach-to-Covid-19.pdf.
- Jackson LA, Anderson EJ, Rouphael NG et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. New England Journal of Medicine. 2020;383(20):1920-1931. https://www.nejm.org/doi/full/10.1056/NEJMoa2022483.
- Allen A, Szabo L. NIH “very concerned” about serious side effect in coronavirus vaccine trial. Scientific American, September 15, 2020. https://www.scientificamerican.com/article/nih-very-concerned-about-serious-side-effect-in-coronavirus-vaccine-trial/.
- Mayer A. Leading COVID vaccine candidates plagued by safety concerns. The Defender, November 13, 2020. https://childrenshealthdefense.org/defender/covid-vaccine-candidates-safety-concerns/?itm_term=home. .
- U.S. Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee, October 22, 2020 Meeting Presentation, slide #16. https://www.greenmedinfo.com/blog/covid-19-vaccine-bombshell-fda-documents-reveal-death-21-serious-conditions-possi1.
- Reals T. U.K. warns against giving Pfizer vaccine to people prone to severe allergic reactions. CBS News, December 9, 2020. https://www.cbsnews.com/amp/news/covid-vaccine-pfizer-shot-uk-warning-people-with-history-of-significant-allergic-reactions/#app.
- https://www.fda.gov/media/144245/download, page 42.
- Public Readiness and Emergency Preparedness Act. COVID-19 PREP Act Declarations. https://www.phe.gov/Preparedness/legal/prepact/Pages/default.aspx.
- Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. Journal of Translational Autoimmunity. 2020;3:100051. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7142689/.
- Khemlani A. Fauci: Early COVID-19 vaccines will only prevent symptoms, not block the virus. Yahoo! Finance, October 26, 2020. https://finance.yahoo.com/news/fauci-vaccines-will-only-prevent-symptoms-not-block-the-virus-195051568.html.
- Scipioni J. Dr. Fauci says masks, social distancing will still be needed after a Covid-19 vaccine—here’s why. CNBC, November 16, 2020. https://www.cnbc.com/2020/11/16/fauci-why-still-need-masks-social-distancing-after-covid-19-vaccine.html.
- Centers for Disease Control and Prevention. COVID-19 pandemic planning scenarios. Updated September 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. 15.Petition/motion for administrative/regulatory action regarding confirmation of efficacy end points and use of data in connection with the following clinical trials. Dr. Wolfgang Wodarg and Dr. Michael Yeadon, petitioners. Filed with European Medicines Agency, December 1, 2020. https://healthimpactnews.com/wp-content/uploads/sites/2/2020/12/Wodarg_Yeadon_EMA_Petition_Pfizer_Trial_FINAL_01DEC2020_EN_unsigned_with_Exhibits.pdf.
Please get vaccinated and help stop the spread of FUD, as well as SARS-CoV2. If you believe there are mistakes or mischaracterizations in this post, please email me at covid19@tylerbenster.com, and I’ll do my best to fix or clarify.
← Back to Writing